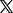

Microspheres that sense gastrointestinal disease are suspended in solution (L), and then attracted to the side of a test tube by a magnet (R) so researchers can easily retrieve them from biological samples. A study published this week touts the process as a possible alternative to invasive colonoscopies for detecting GI diseases. Photo courtesy of the American Chemical Association
Chinese researchers say a new kind of bacteria-packed pill can quickly detect gastrointestinal disease and has the potential to replace invasive colonoscopies in screening for ulcerative colitis and other gut maladies.
The results of a mouse study published this week in an American Chemical Society journal are raising hopes that timely and accurate diagnosis of inflammatory bowel diseases such as colitis and Crohn’s disease could be performed non-invasively in the future.
Colonoscopies, in which a flexible tube fitted with a camera is inserted into the rectum, are now considered the gold standard for IBD detection and are prized for their ability to provide direct visualization of the lining of the bowel and to find any intestinal bleeding associated with colitis or Crohn’s.
And even though doctors warn the procedures are especially vital in helping to avoid intestinal surgery, many patients dread them — including the preparation — as uncomfortable and time-consuming.
Recent advances, however, may help those with IBD, which is estimated to affect between 2.4 and 3.1 people per million in the United States.
Scientists are developing an understanding of how IBD could be treated by “bacterial therapy,” in which certain types of bacteria present in a patient’s gut microbiome could be enhanced or suppressed.
They’ve also come up with a way to spot telltale biomarkers for IBD by using specially engineered strains of e. coli bacteria that “light up” when exposed to blood in the gut. The question has been how to make them more “bioavailable,” or effectively delivered to their target.
The researchers responsible for this week’s study employed such bacteria in a newly developed platform they call “MagGel-BS,” consisting of miniature pills packed with tiny microcapsules embedded in a biocompatible hydrogel.
Inside the capsules are bacteria capable of detecting intestinal bleeding and, in a new innovation, they are accompanied by magnetic particles which allow doctors to quickly remove the sensors from feces after passing through the gastrointestinal system.
Results showed the MagGel-BS biosensors were capable of confirming the presence of gastrointestinal bleeding just 20 minutes after their extraction, a “significant improvement” over previous efforts using unencapsulated biosensors that took several hours to yield results.
Importantly, their use produced no immune responses or adverse effects in the mice.
The authors thus deemed the process “an efficient and rapid approach for gut disease detection, advancing the potential for bacterial therapies in clinical applications.”
Study co-author Ying Zhao, a professor of biochemical engineering at the East China University of Science and Technology in Shanghai, told UPI in emailed comments the most exciting thing about the results is how they demonstrate the leaps made in biosensor technology.
“While the encapsulation of probiotics in hydrogels to improve their bioavailability is a well-established technology, embedding both bacterial sensors and magnetic particles into hydrogel beads for gut disease detection presents an innovative solution,” she said.
“Advances in bacterial sensor engineering have enhanced their sensitivity, speed, and precision, improving their performance in practical disease diagnostics.”
The platform, if proven safe and effective for humans, “could offer a major advancement in non-invasive diagnosis for gastrointestinal diseases like colitis and colorectal cancer,” Ying added.
“By selecting the right engineering bacterial sensor, this ‘plug-and-play’ technology could enable at-home testing, providing a non-invasive alternative to colonoscopy,” she said.
While still in the very early stages of development, the new platform has piqued the interest of experts in the IBD field.
“The idea of using engineered bacteria as ‘sensors’ for intestinal disorders is an interesting concept,” said Dr. Alan Moss, chief scientific officer for the nonprofit Crohn’s & Colitis Foundation, which since its founding in 1967 has invested more than $500 million into finding the causes, treatments and cures for Crohn’s disease and ulcerative colitis.
“Current gold standards, such as fecal calprotectin and colonoscopy, have limitations for monitoring patients with Crohn’s disease or ulcerative colitis,” he told UPI. “Tools like those reported in this paper could provide a method to track more specific biomarkers of intestinal disease in real time.”
However, Moss cautioned, “results in mice do not always translate directly to humans, so further studies will be needed to demonstrate the safety and utility of bacterial sensors in real-world settings.”